Biologics
Biologics
This chapter is prepared for PharmD students in accordance with the officially endorsed curriculum in Pakistan.
August 27, 2023
Chapter outline
» Introduction
» Classification of biologics
» Immunity
» Vaccines
» Toxins, toxoids, and antitoxins
» Venoms and antivenins
» Antiserums
» Plant-based biologics
Introduction
Biologics means any product derived from a living plant or animal source.
However, strictly interpreted, the term biologics refers to ‘any virus, therapeutic serum, toxin, antitoxin, or analogous product,’ and it has been interpreted to include a lengthy list of such products as vaccines of bacterial, rickettsial, and viral origin, immune serums for the prevention or treatment of disease, various miscellaneous and diagnostic products, human blood, and products derived from human blood.
Such substances as insulin, liver extract, and antibiotic products are not classified as biologics.
The broad term ‘biologics’ thus includes the immunizing biologicals which are derivatives of animals (serums, antitoxins, and globulins) or of microscopic plant organisms (vaccines, toxins, toxoids, and tuberculins), that either directly or indirectly confer a state of protection against pathogenic microorganisms.
Since these products don’t affect the microorganisms directly, they cannot be considered chemotherapeutic agents; neither can they be classified with antibiotics.
Classification of biologics
» Biologics can be classified into two general categories;
1. Antigens
2. Antibodies
1. Antigens
» An antigen is a molecule or a molecular structure or any foreign particulate matter or a pollen grain which induces an immune response in the body, especially the production of antibodies
» An antigen can be defined under three categories, i.e., biological, chemical, and physical
» Biologically, it is a substance, which when introduced into the tissues of man or other vertebrates will cause the formation of antibodies
› Antigen possesses the following biological properties;
› Immunogenicity: The capacity to induce antibody formation
› Specificity: It is governed by small chemical sites on the antigen molecule called the ‘antigenic determinants’ (this is the site with which antibody combines)
› Considered foreign: The antigen must be considered foreign by the antibody forming host
» Chemically, antigens are usually proteins; however, some high molecular weight polysaccharides are antigenic
» Physically, antigens must possess high molecular weight, i.e., more than 10,000
› The high molecular weight is associated with immunogenicity
» Examples of antigens are;
› Exotoxins
› Proteins and polysaccharides on the cell surface and capsules of bacteria
› Protein coat of virus particles
› Microorganisms contain not one but many antigens, which, in turn, may contain many antigenic determinants
2. Antibodies
» An antibody is a blood protein, produced in response to and counteracting a specific antigen
» Antibodies are produced by plasma cells (a type of white blood cell)
» Antibodies are found predominantly in the serum fraction of blood
» They also exist in other body fluids and in association with other tissues like lymph nodes and mucous membrane
» When serum proteins are separated by electrophoresis, the following four predominant fractions are obtained
› 1. Serum albumin
› 2. Alpha globulin
› 3. Beta globulin
› 4. Gamma globulin
› Antibodies occur in the ‘gamma globulin’ fraction and, therefore, are called immunoglobulins
Classification of antibodies (immunoglobulins)
» Classification is based on the physical, chemical, and immunological properties
› Immunoglobulin A (IgA)
› Immunoglobulin D (IgD)
› Immunoglobulin E (IgE)
› Immunoglobulin G (IgG)
› Immunoglobulin M (IgM)
» IgG accounts for 70-75% of all human immunoglobulins found in the blood
› IgG is the main component of the humoral immune system (immune response initiated by macromolecules present in the extracellular fluid) because of its abundance
› IgG is the only antibody that can cross the placenta and provides passive immunity to the fetus and infants in the first few months of life
» IgM is the largest antibody and the first one to be synthesized in response to an antigen or microbe
› IgM accounts for 5% of all immunoglobulins present in the blood
› IgM typically exists as polymers of identical subunits, with a pentameric form as the prevalent one
› Due to its large size, IgM is mostly intravascular and has a lower affinity for antigens
› However, since pentameric IgM has 10 antigen binding sites, it has higher avidity (overall binding strength) for antigens than IgG and acts as an excellent activator of the complement system and agglutination
» IgA accounts for 10-15% of all immunoglobulins
› IgA is prevalent in serum, nasal mucus, saliva, breast milk, and intestinal fluid
› At mucosal surfaces, IgA provides the primary defense against inhaled and ingested pathogens
» IgE is the least prevalent one, with a serum concentration 10,000 times lower than IgG
› However, the concentration of IgE increases significantly in allergic conditions, such as bronchopulmonary aspergillosis, and parasitic diseases, such as schistosomiasis
» IgD functions as a B cell antigen receptor and may participate in B cell maturation, maintenance, activation, and silencing
› Although the exact function is still unclear, IgD may be involved in humoral immune responses by regulating B cell selection and homeostasis
Haptens
» Compounds with a molecular weight lower than 10,000, are partial antigens, called haptens
» Due to low molecular weight, they lack the property of immunogenicity
» However, they can attach to host proteins to form a complete antigen
» Example: Drugs or their breakdown products may act as haptens, e.g., penicillin allergy
Immunity
» Immunity is the state or quality of being resistant to a particular infectious disease or pathogen
» Immunity is the immune system’s way of protecting the body against an infectious disease
» Immunity is classified into 2 major types;
1. Innate (natural) immunity
2. Acquired immunity
» Acquired immunity is generally subdivided into 2 classes;
A. Active immunity (i. naturally acquired; ii. artificially acquired)
B. Passive immunity (i. naturally acquired; ii. artificially acquired)
1. Innate (natural) immunity
» The term natural or innate means the defense mechanisms that are present in the body because of race, species specificity, and a multitude of other factors not easily defined
» It does not include any mechanisms especially developed during the lifetime of the individual
» Natural immunity is endowed at birth and is retained because of an individual’s constitution
2. Acquired immunity
» Acquired immunity is a type of immunity that develops when a person’s immune system responds to a foreign substance or microorganism, or that occurs after a person receives antibodies from another source
» Acquired immunity is quite specific
» Acquired immunity is generally subdivided into 2 classes;
A. Active immunity
B. Passive immunity
A. Active immunity
» Active immunity means the specific immunity developed by an individual in response to the introduction of antigenic substances into the body
» Active immunity is developed gradually/slowly
» Active immunity is usually long-lasting
» Active immunity can be divided into the following two categories;
i. Naturally acquired active immunity
» In this type of immunity, the antigenic substances are received by the body in a natural manner
» For example, recovery from an infection, such as measles or scarlet fever, produces an immunity
» This immunity is acquired naturally
ii. Artificially acquired active immunity
» In this type of immunity, the antigenic substances are received by the body through the administration of a vaccine or toxoid
» This immunity may be produced as the response to a series of injections
» Thus, stimulating the body cells to make their own antibodies and producing an immunity
» For example, the typhoid vaccine produces active immunity
» This immunity is acquired artificially
B. Passive immunity
» Passive immunity is the type developed by the introduction of preformed antibodies (not antigens) into the body
» In this type, the body cells are not stimulated to produce their own antibodies
» This immunity is produced quickly
» Passive immunity is not long-lasting
» Passive immunity can be divided into two categories;
i. Naturally acquired passive immunity
» The immunity developed in a newborn infant through the transmission of the antibodies from the blood of the mother
ii. Artificially acquired passive immunity
» The injection of immunizing biologics containing preformed antibodies in forms produces artificially acquired passive immunity
» Examples: Mumps immune globulin, antirabies serum, etc.
Recall or anamnestic phenomenon
» In the case of active immunity, depending on the nature of the antigen and the site of injection, antibodies can be detected in the serum several days after the first injection of antigen
» The antibody titer rises gradually to a low peak after the first injection (primary response)
» The antibody titer rises immediately after subsequent (second, third, etc.) injections
» The antibody titer then falls slowly over a period of months
» A second injection of antigen, administered while antibodies from the first stimulus are still present, results in a rapid rise to a much higher peak than with the first injection (secondary response)
» The second injection should not be too close in time to the first injection
› If so, there is no additional effect on antibody production
» The antibodies disappear much more slowly after the second stimulus than after the first
» There is rapid rise of antibody titer following a second administration of the antigen (the booster shot)
» It presumably indicates that the antibody-producing cells have been primed by the first contact with antigen
› Therefore, respond more effectively and more quickly when they encounter the antigen a second time
» So, immune cells recognize the invading substance (an antigen) and produce antibodies specific against that antigen
» During the immune response occurring on the second and subsequent exposures to an antigen, the peak antibody titer is higher, lasts longer, and is achieved rapidly, compared to a primary response and this phenomenon is termed the ‘recall’ or ‘anamnestic phenomenon’ (Figure 1)
» The major cellular components of the immune system are the macrophages and the lymphocytes
» Certain lymphocytes, called B cells, produce antibodies
› B cells arise from the bone marrow in humans
» Immunity by B cells (production of antibodies) is called humoral system of immunity because the B cells circulate in the body fluids, primarily in blood
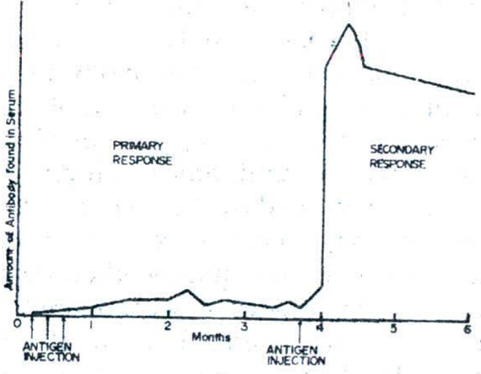
Figure 1: An illustration of the ′recall′ or ′anamnestic phenomenon′ in antibody production
Vaccines
» A vaccine is a preparation containing weakened or dead microbes of the kind that cause a particular disease
» Vaccines are administered to stimulate the immune system to produce antibodies against that disease
» Vaccines may contain living, attenuated, or killed viruses, killed rickettsiae, or attenuated or killed bacteria
» Vaccines are available in dried and in liquid form
» Primary active immunity from vaccination develops more slowly than the incubation period of most infections
› Hence, vaccines must be induced prior to exposure to the infectious agent
› Therefore, the general action of vaccines should be considered prophylactic
» One exception is the rabies vaccination
› The rabies virus has a median incubation period of 35 days in humans
› There is usually sufficient time for protective antibodies to develop when the vaccine is administered after exposure
» Nonliving vaccines provide protection for only a limited time
› Therefore, repeated vaccination is required
› For example, to maintain protection against typhoid fever, cholera, plague, and typhus
» Active immunization with living agents is generally preferable to immunization with killed vaccines
› They have a superior and more long-lived immune response
› For example, a single vaccination of measles, rubella, or mumps vaccine is sufficient to produce a long-lasting immunity
» Use of vaccines is contraindicated under conditions in which the immune response may be depressed
› For example, during therapy involving corticosteroids, antineoplastic agents, immunosuppressive agents, or radiation
› In patients with immunoglobulin deficiency
» Active immunization may cause fever, malaise, and soreness at injection sites
» Allergic reactions may also occur
› As a result of either from the organism constituting the vaccine or from a protein incorporated into the vaccine during manufacture
Viral vaccines
» Viral vaccines for prophylaxis against mumps, rubella, rubeola, smallpox, and yellow fever contain living viruses
» Inactivated or killed viruses are used in influenza and rabies vaccines
» Preparations containing live attenuated or killed viruses are available for immunization against poliomyelitis
» The cultivation of viruses poses a problem because they are completely dependent on living cells for their sustenance
› No method of growing viruses in artificial culture media is known
» A number of viruses currently employed in viral vaccines are grown on tissue cultures prepared from chick embryo, monkey kidney, or human diploid cells
Smallpox vaccine
» Smallpox vaccine is the living virus of vaccinia (cowpox) that has been grown in the skin of a vaccinated bovine calf
» It is available in dried and in liquid form
» Liquid vaccine should be stored below 0 °C
» Dried vaccine between 2 °C and 8 °C
Source
» Living virus of vaccinia (cowpox), grown in the skin of a vaccinated bovine calf
Historical background
» The pioneer work of Dr. Edward Jenner established that when a mild case of cowpox is developed by a person, the same person becomes immune to smallpox
» Using this information, he inoculated a young boy with pus from a milkmaid, infected with cowpox
» Two months later, the boy was inoculated with pus from a patient infected with smallpox, but no disease developed
» Immunity had been established
Preparation
» Belly of the calf is washed and shaved
» Epidermis is scarified, so that serum oozes through the cuts
» The ‘seed virus’ is inoculated into the scarifications by hand rubbing
» Workers are protected by rubber surgical gloves
» Calf is maintained in an aseptic stall
» Calf is given food and water during the growth of the virus
» The vesicles that develop, are removed, thoroughly triturated, and
› Either made into a smooth suspension with an aqueous solution of glycerin or sorbitol, or
› reduced to a dried pellet
» The animal must be in good health prior to inoculation
» After the virus is harvested, the animal is killed and a necropsy is performed
» If the organs show no effects of disease from other causes, the virus is deemed satisfactory for manufacture
Uses
» It is used as prophylactic against smallpox
» It develops active immunity which lasts for about 7 years
Dose
» Percutaneous, contents of 1 capillary tube, by multiple puncture method
» Routine immunization against smallpox is no longer recommended
› Because of severe adverse reactions
› Because the disease has now undergone complete worldwide eradication
» Smallpox vaccination is now indicated only for laboratory workers directly involved with the virus
» Dryvax®
Rabies vaccine
» Rabies vaccine, also known as human diploid cell rabies vaccine (HDCV), is a sterile lyophilized preparation of either the whole virion or subvirion rabies virus
» Both vaccines are supplied as 1 mL, single-dose vials of lyophilized vaccine with accompanying diluent
Source
» The whole virion vaccine is prepared from Wistar rabies virus
› Grown in cultures of human diploid embryo lung tissue, and
› Inactivated with tri-N-butyl phosphate and β-propiolactone
» The subvirion vaccine is prepared from the Pasteur-derived Pitman-Moore virus
› Grown on human diploid cell cultures developed in Europe, and
› Inactivated with β-propiolactone
Historical background
» Louis Pasteur is associated with rabies
» L. Pasteur was able to ‘fix’ the virus of rabies, by
› Passing it from an infected dog to the brain of a rabbit, and
› Then from one rabbit to another, until a uniformity was established, that
› Resulted in attenuated virulence for humans
» Pasteur used such a ‘fixed virus’ to achieve active immunity
» This treatment is not a curative treatment
› It actually causes the immunization of a patient, bitten by a rabid animal, to develop more quickly (by producing antibodies) than the incubation period of the disease
› So, the growth of the rabies virus is inhibited
Uses and dose
» The vaccine is an active immunizing agent
» Recommended primarily for the prevention of rabies in persons, bitten by an animal, supposed or known to be rabid
» However; the vaccine may be used for pre-exposure immunization for veterinarians or other high-risk individuals
» The usual pre-exposure dose is 3 injections of 1 mL of reconstituted vaccine
› On each of days 0, 7, and 21
» Post-exposure immunization should be started as quickly as possible after the wound has been inflicted
› The usual administration schedule is 5 injections of 1 mL of reconstituted vaccine
› On each of days 0, 3, 7, 14, and 28
» Administered intramuscularly (IM)
» Rabies immune globulin should be administered at the time of the first dose of vaccine for additional protection, particularly in case of a bite from wild animal
» Imovax® rabies vaccine
» Wyeth® rabies vaccine
» Formerly, the vaccine was prepared by growing the virus in the brain tissue of rabbit
» Brain tissue of rabbit contains a significant amount of myelin
» Myelin causes paralysis
» With the advent of HDCV, problems with paralysis have been greatly reduced
» Rare cases of Guillain-Barre syndrome have been reported
› However, these patients recovered completely from the paralysis
Guillain-Barre syndrome is a disorder in which your body’s immune system attacks your nerves.
Yellow fever vaccine
» Yellow fever vaccine is an attenuated strain of living yellow fever virus, selected for high antigenic activity and safety
» Should be stored at a temperature preferably below 0 °C but never above 5 °C
Source
» Yellow fever virus, grown in the living embryo of domestic fowl, Gallus domesticus
Preparation
» The virus infected chick embryo pulp is suspended in water and after appropriate aseptic processing, is distributed in suitable quantities into ampules and dried from the frozen state
» The ampules are filled with dry nitrogen and flame sealed
Historical background
» Yellow fever or ‘yellow jack’ was considered an endemic disease in certain tropical regions, including Central America
» Work on the Panama Canal was abandoned by the French because of the terrific death toll caused by yellow fever
» Through numerous volunteers among the American troops, stationed in Cuba (during the Spanish-American war), the Aedes mosquito was finally proved to be the vector of the disease
Uses
» Active immunizing agent against yellow fever
» 0.5 mL, subcutaneous
» YF-VAX®
» Stamaril®
Influenza virus vaccine
» Influenza virus vaccine is a sterile aqueous suspension of suitably inactivated influenza virus types A and B, either individually or combined, or
› Virus subunits prepared from the extra-embryonic fluid of influenza virus-infected chick embryo
» The strains of influenza virus used in the preparation of this vaccine are those designated for the particular season by the Center for Drugs and Biologics of the Federal FDA
» Influenza viruses have a high degree of strain specificity and genetic instability
› These factors require a continual re-evaluation of the components of the influenza virus vaccine and result in periodic infections of epidemic proportions even among immunized persons
» Most available vaccines are bivalent and contain types A and B virus strains
» Should be stored at a temperature between 2 °C and 8 °C
Source
» Inactivated influenza virus; grown in the chick embryo
Preparation
» The virus growths are collected, concentrated, refined by ultracentrifugation, and inactivated by UV radiation
Uses
» Active immunizing agent against influenza
» Intramuscular (IM); 0.5 mL
» Annual vaccination is recommended for individuals in high-risk categories e.g., those who are immunocompromised, those over 65 years of age, etc.
» Fluarix® (GSK)
» Fluogen®
Poliomyelitis vaccine
» Polio is caused by a human enterovirus called the poliovirus
» The virus is most often spread by the fecal-oral route
» Poliovirus enters through the mouth and multiplies in the intestine
» Infected individuals shed poliovirus into the environment for several weeks, where it can spread rapidly through a community, especially in areas of poor sanitation
» Poliomyelitis can affect any age, but primarily involves children ages less than 5 years and can cause paralysis or even death
Poliomyelitis vaccine
» The first polio vaccine to be widely used in humans, known as inactivated poliovirus vaccine (IPV) or Salk vaccine, was developed in the early 1950s by American physician Jonas Salk
› This vaccine contains killed virus and is given by injection
› The large-scale use of IPV began in 1954, when it was administered to American schoolchildren
› In the following years, the incidence of polio in the United States fell from 18 cases per 100,000 people to fewer than 2 per 100,000
» In the 1960s, a second type of polio vaccine, known as oral poliovirus vaccine (OPV) or Sabin vaccine, was developed (named for American physician and microbiologist Albert Sabin)
› OPV contains live attenuated (weakened) virus and is given orally
» OPV is administered orally and does not require health professionals or sterile needle syringes
» OPV is easy to administer in mass vaccination that is why used in polio campaigns that take place in Pakistan
» Moreover, for several weeks after vaccination the vaccine virus replicates in the intestine, is excreted, and can be spread to others in close contact
» This means that in areas with poor hygiene and sanitation, immunization with OPV can result in passive immunization of people who have not been vaccinated
» IPV is an extremely safe vaccine and highly effective in protecting children from polio
» It produces antibodies in the blood against poliovirus
» Unlike OPV, IPV has limited ability to stop the spread of virus in a community
» This is why in the polio-endemic countries such as Pakistan, OPV is the predominant vaccine used in the fight to eradicate the virus
» IPV requires trained health workers, as well as sterile injection equipment and procedures
» Combining OPV and IPV provides stronger protection against polio
› IPV strengthens immunity in the blood while OPV strengthens immunity in the gut
» IPV has been introduced into the routine schedule across Pakistan to give children the best protection against polio
› The current routine immunization schedule recommends one dose of IPV and multiple doses of OPV for full protection against polio
› Once polio is eradicated, IPV will be the only vaccine available for routine use
Source
» Poliomyelitis virus strains (type 1: Mahoney strain; type 2: MEF-1 strain; type 3: Saukett strain); grown separately in cultures of Rhesus monkey kidney tissue
» In addition to the 3 types of poliomyelitis virus that have been cultured and identified, other paralysis-producing strains undoubtedly exist
» Immunization with one type of virus does not offer protection against the other types
› Thus, the current vaccine is a trivalent preparation
Preparation
» To prepare Salk vaccine, the virus strains are grown separately in cultures of Rhesus monkey kidney tissues, bathed by a complex nutrient fluid containing more than 60 ingredients
» After incubation, the virus is harvested by decanting the nutrient fluid that is clarified by filtration
» Then, formaldehyde is added
» Formaldehyde treated viruses are maintained at 36 °C and pH 7, until all viruses are killed
» A series of tests are performed to ascertain that all viruses are inactivated
» Then the formaldehyde is neutralized and a preservative is added
» The 3 types of viruses are then pooled, the resultant mixture is the trivalent vaccine
» For the preparation of Sabin vaccine, the method of preparation is same as that for Salk polio vaccine
› The difference is that, in this vaccine, virus strains are not killed by treatment with formaldehyde, instead the viruses are attenuated
Uses and dose
» To develop active immunity against poliomyelitis
» Salk vaccine provides protection against paralytic poliomyelitis through the stimulation of serum antibodies specific for types 1, 2, and 3 poliovirus
› But, does not cause the inhibition of viral growth in the intestine that characterizes the Sabin vaccine
» Sabin oral vaccine should never be administered parenterally
» Orimune®
» Poliovirus vaccine live oral is generally frozen
› When stored at a temperature of -10 °C, the expiration date is not later than 1 year, after the date of manufacture or date of issue
› It may be thawed and refrozen not more than 10 times, provided that the thawed material is kept refrigerated and the total cumulative duration of the thaw is not more than 24 hours
Measles vaccines
» Vaccines containing live attenuated rubeola (measles) and rubella (German measles) viruses
Rubeola (measles) vaccine
» Measles virus vaccine live or rubeola vaccine is prepared from attenuated viruses derived from the original Edmonston B strain or the Enders strain
» The Enders strain is a modified Edmonston strain, and it is claimed to have a high degree of antigenicity with a low incidence of adverse reactions
› Coadministration of immune globulin may not be necessary with vaccines employing this strain
» The rubeola virus is grown on cultures of chicken embryo tissue
» The vaccines are available in a lyophilized form
» Should be stored at a temperature between 2 °C and 8 °C
Uses and dose
» Rubeola vaccine is recommended for active immunization of children 15 months of age or older
» Use in infants under 15 months of age is not recommended
» Good immunity is obtained with a single subcutaneous injection of not less than 1000 TCID50 (tissue culture infectious doses) of the reconstituted vaccine
The TCID50 is the quantity of virus estimated to infect 50% of inoculated tissue cultures.
» Attenuvax®
Rubella (German measles) vaccine
» Rubella virus vaccine live is prepared from the Wistar Institute RA 27/3 strain grown on human diploid cell tissue
» Rubella vaccine is recommended for active immunization against German measles for children aged 1 to puberty and for certain other individuals
» This vaccine should not be administered to pregnant or immediate postpartum women
» Special caution must be exercised if it is given to sexually active females
» Precautions must be taken to eliminate the possibility of pregnancy in women of child-bearing age for at least 3 months following immunization
Uses and dose
» Immunity is obtained with a single subcutaneous injection of not less than 1000 TCID50 of the reconstituted vaccine
» Use in infants under 1 year of age is not recommended
» Meruvax II®
Mumps vaccine
» Mumps vaccine is the vaccine containing live attenuated mumps virus
Source
» B-level Jeryl Lynn strain of the virus; grown in the cell culture of chicken embryo
Uses and dose
» To produce active immunity against mumps
» It provides active immunity for at least 10 years after immunization
» It is particularly valuable to susceptible individuals approaching puberty and to adults
» It is not recommended for infants less than 1 year old
› Because they may retain maternal mumps antibodies that may interfere with the immune response
» The vaccine is available in a lyophilized form
» Immunization involves a single subcutaneous injection of not less than 5000 TCID50 of mumps virus vaccine
» Mumpsvax®
Combination virus vaccines
» Combination live virus vaccines are available
› Measles + Rubella
› Mumps + Rubella
› Measles + Rubella + Mumps
» These combination vaccines are administered subcutaneously to children 15 months of age or older
» Use in infants under 15 months of age is not recommended
» Priorix® (GSK) [Measles + Mumps + Rubella]
» M-M-R II® (MSD) [Measles + Mumps + Rubella]
Hepatitis vaccine
» Hepatitis B vaccine is composed of chemically inactivated hepatitis B surface antigen (HBsAg) particles
» HBsAg particles are obtained from the plasma of healthy chronic HBsAg carriers
» Specific antibody (anti-HBs) develops in 75 to 90% of healthy adults after the first 2 doses of vaccine; and in 85 to 90% after the third dose
» Vaccine-induced antibody has persisted for at least 3 years
» Booster doses are also required
Uses and dose
» Vaccination is recommended for individuals in high-risk categories
» The vaccine is given intramuscularly (IM)
› 3 doses of 1 mL (20 μg)
› The first 2 doses are one month apart, and
› A booster dose, administered 6 months after the first dose
» For patients on dialysis and others who are immunocompromised
› 3 doses of 2 mL (40 μg) should be used
» For children under 10 years old
› 3 doses of 0.5 mL (10 μg) are recommended
» Engerix B® (GSK)
» Euvax-B®
Rickettsial vaccines
» Rickettsial diseases are a group of diseases with similar symptoms caused by bacteria of the genus Rickettsia
» The rickettsiae are a diverse collection of intracellular Gram-negative bacteria
› Found in ticks, lice, fleas, mites, and mammals
» These bacteria are spread by the bite of an infected tick or mite
Typhus vaccine
» Typhus vaccine is a sterile suspension of the killed rickettsial organism Rickettsia prowazekii, selected for antigenic efficiency
» Should be stored at a temperature between 2°C and 8°C
Source
» Rickettsia prowazekii; grown in the yolk sac membrane of the developing embryo of Gallus domesticus
Uses
» To develop active immunity against epidemic typhus fever
» Subcutaneous; 2 injections of 0.5 mL, 4 or more weeks apart
› Followed by 0.5 mL every 6 to 12 months, as long as protection is required
Rocky Mountain spotted fever vaccine
» Rocky Mountain spotted fever vaccine is a sterile suspension of the killed rickettsial organism Rickettsia rickettsii, selected for antigenic efficiency
» Should be stored at a temperature between 2 °C and 8 °C
Source
» Rickettsia rickettsii; grown in the yolk sac membrane of the developing embryo of Gallus domesticus
Uses
» To develop active immunity against rocky mountain spotted fever
» Subcutaneous; 3 injections of 1mL, each at 7 to 10 days interval
» An annual 1mL booster injection is highly recommended
Bacterial vaccines
» Bacterial vaccines consist of suspensions of attenuated or, more commonly, killed pathogenic bacteria in isotonic sodium chloride solution or other suitable diluents
» The strains of bacteria employed in the preparation of the vaccines must be selected for high antigenicity
» A measure of the potency of a vaccine may be expressed as the number of organisms per unit volume or as biologic reference units
» Suspensions of young, living organisms grown in standard culture media are killed
› Chemically
› By application of moist heat at a temperature slightly above the thermal death point, or
› by exposure to ultraviolet light
» The smooth or ‘S’ strains of bacteria are uniformly more antigenic than the rough or ‘R’ strains
» Occasionally, stock cultures lose their antigenic qualities, and care must be exercised in a biologic manufacturer’s laboratory to ensure the use of suitable strains
Typhoid vaccine / Enteric vaccine
» Typhoid vaccine is a sterile suspension containing killed typhoid bacilli Salmonella typhi
» It contains approximately 1 billion typhoid organisms in each mL
» Typhoid vaccine has been called enteric vaccine because it prevents the effect of the disease on the intestinal tract
Source
» Killed typhoid bacilli Salmonella typhi
Uses
» To produce active immunity against typhoid fever
» It is recommended for persons who have had household contact with a known, typhoid carrier, or
› For travelers going to areas of the world where typhoid fever is endemic
Dose
» Subcutaneously
› 2 injections of 0.5 mL, 4 weeks apart
› Followed by 0.5 mL every 3 years thereafter
» Booster injection is recommended when the danger of typhoid occurs
» Typherix® (GSK)
Cholera vaccine
» Cholera vaccine is a sterile suspension containing killed cholera vibrio Vibrio cholerae
Source
» Equal portions of suspensions of killed cholera vibrios (Vibrio cholerae) of the Inaba and Ogava strains
» It contains approximately 8 billion cholera organisms in each mL
Uses
» To produce active immunity against cholera
» Administered orally
› 2 doses for adults and children from 6 years of age
› Children 2 to below 6 years of age should receive 3 doses
› Doses are to be administered at intervals of at least one week
» Dukoral® (Crucell)
Plague vaccine
» Plague vaccine is a sterile suspension containing killed plague bacilli Yersinia pestis
» Rats serve as an animal reservoir for the organisms, but the disease is transmitted to humans through the bites of fleas that infest the rats
» With rat control and largescale vaccination, plague can be eliminated
Source
» Killed plague bacilli Yersinia pestis
» It contains approximately 2 billion plague organisms in each mL
Uses
» To produce active immunity against plague
» Its use is generally restricted to travelers to Southeastern Asia and to persons who have frequent contact with wild rodents
» IM; 2 injections of 0.5 ml, 4 weeks apart; Then 0.2 ml 4 to 12 weeks later
Pertussis vaccine
» Pertussis is also called whooping cough
» Cough is caused by a toxin in the bacterial body
» The organisms attach themselves to the cilia of epithelial cells in the trachea and the irritation produced provokes the cough spasm
» Pertussis vaccine is a sterile suspension containing killed pertussis bacilli Bordetella pertussis
» It has a potency of 12 protective units per dose
» Should be stored at a temperature between 2 °C and 8 °C
Source
» Killed pertussis bacilli Bordetella pertussis
Pertussis
» It is also called whooping cough
» Cough is caused by a toxin in the bacterial body
» The organisms attach themselves to the cilia of epithelial cells in the trachea and the irritation produced provokes the cough spasm
Uses
» To produce active immunity against pertussis
» Subcutaneous; 3 injections of 0.5 or 1 ml, 3 to 4 weeks apart
Adsorbed pertussis vaccine
» Pertussis vaccine that has been precipitated or adsorbed by the addition of aluminum hydroxide or aluminum phosphate and resuspended
Uses and dose
» Same as that of the pertussis vaccine
» Route of administration is IM
BCG vaccine
» BCG vaccine is a dried, living attenuated culture of the bacillus Calmette-Guerin (BCG) strain of Mycobacterium tuberculosis var. bovis
» BCG has been used since 1921 globally
Source
» Bacillus Calmette-Guerin (BCG) strain of Mycobacterium tuberculosis var. bovis
Uses and dose
» To produce active immunity against tuberculosis
» Immunological protection against TB is only relative and is not permanent or predictable
» Vaccine is recommended primarily for use for people whose exposure to TB is high
» It should be used only with individuals who give negative tuberculin skin test
» Infants in Pakistan are vaccinated with BCG vaccine because it can prevent severe tuberculosis in children
» BCG vaccine does not provide 100% protection against TB
› It significantly decreases the chances of your baby getting this serious disease
» A single dose of BCG vaccine is administered routinely at birth in Pakistan
» The vaccine is administered just beneath the skin
» If the child misses the dose at birth, it can be administered later on as well
Pneumococcal vaccine
» Pneumococcal vaccine polyvalent affords protection against the 23 most prevalent capsular types of pneumococci, which account for at least 90% of pneumococcal disease
» it is prepared by isolating and purifying the polysaccharide antigens from strains of Streptococcus pneumoniae that contain these serotypes
Uses and dose
» Use of this vaccine is indicated for those 2 years of age or older in whom there is an increased risk of morbidity and mortality from pneumococcal pneumonia
» Even with current antibiotic therapy, the mortality rate in high-risk patients hospitalized with pneumococcal infection has remained higher than 25%
» The vaccine is administered as a single 0.5 mL dose given either subcutaneously or intramuscularly
» Severe local reactions have occurred after a second dose
» Therefore, more than one dose is not recommended, even for patients who received an older vaccine that contained fewer pneumococcal types
» Pneumovax®
Haemophilus vaccine
» Haemophilus b polysaccharide vaccine is composed of the purified, capsular polysaccharide of Haemophilus influenzae type b (Hib)
» Virtually all cases of Haemophilus influenzae meningitis among children are caused by strains of Hib
» Despite effective antimicrobial therapy, the mortality rate from Haemophilus meningitis ranges from 5 to 10%
› About one third of the survivors have some form of permanent injury to the central nervous system
» In addition, Hib can cause epiglottitis, osteomyelitis, arthritis, cellulitis, and pneumonia in children
Uses and dose
» Immunization is recommended for all children when they reach 2 years of age and possibly for children 2 to 5 years old who have not been previously immunized
» Most unimmunized children over 5 years old and most adults have protective titers of naturally acquired antibodies
» The vaccine is administered subcutaneously as a single 0.5 mL dose
» Hiberix®
Toxins, toxoids, and antitoxins
» Bacterial toxins are bacterial waste products which are considered poisonous to the animal body
› Toxins act as antigens
› They stimulate the body to produce antibodies, called antitoxins
» An antitoxin is an antibody produced in response to and capable of neutralizing a specific bacterial toxin
› Examples of antitoxins include diphtheria antitoxin, tetanus antitoxin, etc.
» When toxins are excreted from bacterial cells and are dissolved in the surrounding culture, they are referred to as exotoxins
» Endotoxins are toxic substances bound to the bacterial cell wall and released when the bacterium ruptures or disintegrates
› The most common example of endotoxins is lipopolysaccharides (LPS) that are present in the outer plasma membrane of Gram-negative bacteria
» A toxoid is a chemically modified toxin from a pathogenic microorganism, which is no longer toxic
› Toxoid is still antigenic and can be used as a vaccine
› For example tetanus toxoid
Preparation of antitoxins
» Specific bacterial exotoxins are injected into the horse, repeatedly
» Antitoxins, against these injected exotoxins, are produced in the horse
» Blood samples are taken from the horse, tests are performed to check the level of antitoxins
» The horse is bled, the clot is permitted to form, and the clear supernatant serum is separated for processing
» Antitoxins are standardized in terms of antitoxin units
Toxins
Diagnostic diphtheria toxin (Schick test toxin)
» Diagnostic diphtheria toxin is a sterile solution of the diluted, standardized toxic products of growth of the diphtheria bacillus Corynebacterium diphtheriae
Dose
» Intradermal, 0.1 mL
Uses
» Used to determine the susceptibility of the patient to diphtheria
Toxoids
Tetanus toxoid
» Tetanus is a serious but rare condition that can be fatal if untreated
» The bacteria that can cause tetanus can enter our body through a wound or cut in our skin
» These bacteria are often found in soil and manure
» Tetanus toxoid is a sterile suspension of purified toxoid obtained from tetanus bacterium Clostridium tetani
» The steps of its production are; cultivation of tetani bacteria, inactivation (detoxification) of tetanus toxin, purification of tetanus toxoid, mixing of bulk vaccine, and aseptic filling
Uses and dose
» Tetanus toxoid is used to prevent tetanus
» Dose is 0.5 mL intramuscular injection at any age
» Imatet®
Emergency prophylaxis
» Emergency prophylaxis in case of wounds / injuries suspected of tetanus contamination, as follows;
» If tetanus vaccination or revaccination was performed a year or less before the present injury
› Prophylaxis is not necessary
» If tetanus vaccination or revaccination was performed one to five years previously
› A booster dose of 0.5 ml of Imatet must be given intramuscularly
» If vaccination or revaccination was performed more than five years before
› It is necessary to give a booster dose of 0.5 mL tetanus toxoid and also an injection of 1500 IU tetanus antitoxin or tetanus immunoglobulin
» If vaccination or revaccination was not carried out or was incomplete
› Tetanus antiserum as recommended above and a complete course of tetanus vaccination must be performed
Antitoxins
Diphtheria antitoxin
» Diphtheria antitoxin is a sterile, non-pyrogenic solution of refined and concentrated proteins, chiefly globulins, containing antitoxic antibodies obtained from the blood serum or plasma of a healthy horse, that has been immunized against diphtheria toxin or toxoid
» It has a potency of not less than 500 antitoxin units per mL
» Should be stored at a temperature between 2 °C and 8 °C
Source
» Antitoxic antibodies obtained from the blood serum or plasma of a healthy horse, that has been immunized against diphtheria toxin or toxoid
Uses
» To produce passive immunity against diphtheria
» Penicillin and other antibiotics kill the diphtheria organisms, but they have no effect on the toxins
» Any person with clinical symptoms of diphtheria should receive the antitoxin at once without waiting for bacteriologic confirmation
Dose
» IM or IV
» Prophylactic: 1000 to 10,000 units
» Therapeutic: 20,000 to 120,000 units
Tetanus antitoxin
» Tetanus antitoxin is a sterile, non-pyrogenic solution of refined and concentrated proteins, chiefly globulins, containing antitoxic antibodies obtained from the blood serum or plasma of a healthy horse, that has been immunized against tetanus toxin or toxoid
» It has a potency of not less than 400 antitoxin units per mL
» Should be stored at a temperature between 2 °C and 8 °C
Source
» Antitoxic antibodies obtained from the blood serum or plasma of a healthy horse, that has been immunized against tetanus toxin or toxoid
Uses
» Tetanus antitoxin is employed in the treatment and prophylaxis of tetanus, if tetanus immune globulin is not available
» It creates passive immunity to tetanus
» Like diphtheria antitoxin, it is a valuable therapeutic agent when used early in the disease
» Prophylactic doses should be given to individuals who have had 2 or less injections of tetanus toxoid and who have tetanus-prone injuries that are more than 24 hours old
» Tetanus toxoid should also be administered at a different site on the patient
Dose
» Subcutaneous or IM
» Prophylactic: 1500 to 5000 units
» Therapeutic: 50,000 to 100,000 units or more, with at least part of the dose given intravenously
Venoms and Antivenins
» Venom is a poisonous substance secreted by animals such as snakes, spiders, and scorpions and typically injected into prey or aggressors by biting or stinging
Can be compared with exotoxins of bacteria
» Antivenin (antivenom) is an antiserum containing antibodies against specific poisons, especially those in the venom of snakes, spiders, and scorpions
› Example: Anti-snake venom serum (polyvalent equine immunoglobulins)
Preparation of antivenins
» Antivenins are prepared by injecting the specific venoms into the horses, in a manner similar to that for antitoxins
Anti-snake venom serum (Polyvalent equine immunoglobulins)
» Anti-Snake venom serum is a sterile preparation containing purified and concentrated immunoglobulins obtained from the serum of healthy horses immunized against the venoms of the following four common poisonous snakes of Pakistan
Cobra (Naja naja)
Krait (Bungarus caeruleus)
Russell’s viper (Vipera russelli)
Saw scaled viper (Echis carinatus)
Dosage
» Conventionally the dose of anti-snake venom serum is 10-30 mL
» In severe cases, it may go up to 200 mL
» One third of the initial dose can be administered locally around the wound
› Remaining two-thirds of the dose intravenously
» The second dose can be repeated two hours after the first dose or even earlier depending on the condition of the patient and severity of symptoms
» Further doses can be repeated after six hours intervals until the symptoms disappear completely
» The dosage, schedule of repeat dosages, and route of administration of anti-snake venom serum may be modified or adjusted by the specialist/practitioner according to the severity of symptoms
Manufacturer
» Biological Production Division, National Institute of Health, Islamabad, Pakistan
Antiserums
» Antiserum (plural: antisera) is blood serum containing polyclonal antibodies and is used to pass on passive immunity to many diseases
» Examples: Anti-rabies serum, etc.
Preparation of antiserum
» Antiserums are prepared by injecting the specific bacteria or viruses into the horses, in a manner similar to that for antitoxins
» The difference is that, here bacteria or viruses (not toxins) are used for the production of specific antibodies
Antirabies serum
» Antirabies serum is a non-pyrogenic solution containing antiviral substances obtained from the blood serum or plasma of a healthy horse that has been immunized against rabies by means of a vaccine
» New concept in immunization is, the administration of antirabies serum in conjunction with the rabies vaccine
» Now a days, rabies immune globulin is preferred
Uses
Immediate protection against rabies
Dose
IM; 70 units per Kg body weight
Human immune serum globulins (immune globulins)
» A sterile solution of globulins derived from pooled human blood that contains antibodies that are normally present in the blood of adults, used as a passive immunizing agent
» Individuals are hyperimmunized against a particular disease
» Antibodies formed in these individuals are removed
» These are better than antiserums, as chances of sensitization are less
» Examples: Mumps immune globulin, Rho (D) immune human globulin, etc.
Plant-based biologics
» During the last century, the trend upturned and researchers began focusing on biological manufacturing
» Traditional techniques to produce biologics use microbial fermentation or animal cell culture (as discussed in this chapter)
» New concept is ‘plant-based biologics’ or ‘edible vaccines’
» Plant-based biologics have many advantages over conventional biologics, for example;
› Low manufacturing cost
› Manufactured in less time
› Manufacturing can be scaled up easily
› Can be preserved without refrigeration
› No need for ‘cold chains’
› These products are eaten, so no need for trained staff to administer the dose
› Safe, because plant DNA is not known to interact with animal DNA
› Increased safety (lack of contamination with mammalian pathogens)
» To produce such products, the desired genes are integrated into the plants by various methods
› The desired proteins (antigens that act as vaccines) are produced in that plant
› This plant is then eaten or the prepared antigens are isolated and may be administered parenterally
» Some examples are;
» Volunteers ate pieces of potato
› That potato had been genetically engineered to produce part of the toxin secreted by the E. coli bacterium
› These volunteers had four-fold rises in serum antibodies at some point after immunization
» A team of researchers developed genetically modified maize plants
› These plants produce the protein known as HBsAg, which elicits an immune response against the hepatitis-B virus and could be used as a vaccine
» This technology is foreseen to expand its uses in the upcoming years
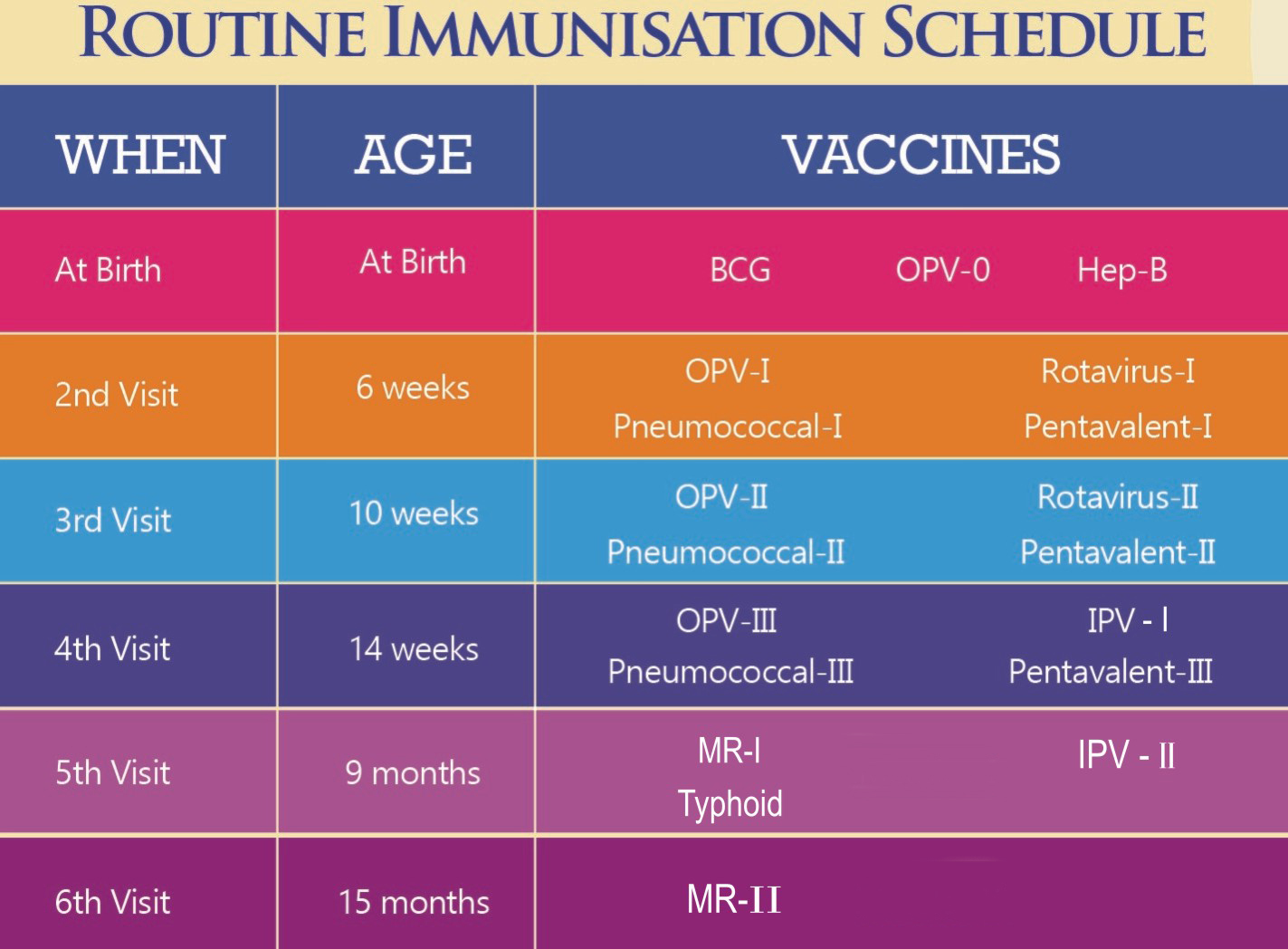
Figure 2: Immunization Schedule in Pakistan. BCG: Bacillus Calmette-Guerin, tuberculosis vaccine; IPV: Inactivated poliovirus vaccine; MR: Measles, mumps, and rubella; OPV: Oral poliovirus vaccine
Reading References
» Bhatia S and Dahiya R. Edible Vaccines. Modern Applications of Plant Biotechnology in Pharmaceutical Sciences, Elsevier, 2015. DOI: 10.1016/B978-0-12-802221-4.00009-1.
» Chen et al. Plant-made biologics. Biomed Res Int., 2014. DOI: 10.1155/2014/418064.
» Laere et al. Plant-based vaccines: production and challenges. Journal of Botany, 2016. DOI: 10.1155/2016/4928637.
» Plant-based biologics: an Effective way to combat life-threatening diseases (www.researchdive.com).
» Tyler VE, Brady LR, Robbers JE. Pharmacognosy. Lea & Febiger, 7th edition, 1976.
» Tyler VE, Brady LR, Robbers JE. Pharmacognosy. Lea & Febiger, 9th edition, 2003.
» www.amson.org.pk.
» www.nih.org.pk.
» www.endpolio.com.pk.
» www.druginfosys.com.
» www.epi.gov.pk.
» www.news-medical.net/life-sciences/Types-of-Antibodies.aspx [Accessed: August 27, 2023].
https://revert2nature.com
